Perspectives Posted on 2020-07-22 17:29:14
Opinions and strategies
African swine fever and the dilemma of a relatively low contagiousness
Keywords
Authors
K. Depner (1)*, K. Dietze (1), A. Globig (1), L. Zani (1), T. Mettenleiter (1) & E. Chenais (2)
(1) Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Greifswald-Insel Riems, Germany.
(2) National Veterinary Institute, Uppsala, Sweden.
* Corresponding author: klaus.depner@fli.de
The designations and denominations employed and the presentation of the material in this article do not imply the expression of any opinion whatsoever on the part of the OIE concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries.
The views expressed in this article are solely the responsibility of the author(s). The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by the OIE in preference to others of a similar nature that are not mentioned.
The anthropogenic factor
Humans are recognised as the main driver of long-distance ASF spread and virus introduction into disease-free populations of domestic and wild pigs. Identifying the anthropogenic or ‘human factor’ is of enormous importance in understanding the pattern of ASF spread. If we consider only the biological characteristics of the disease (e.g. contagiousness, resistance to inactivation and case fatality rate) and neglect the human aspects, we will be unable to control this epizootic [1].
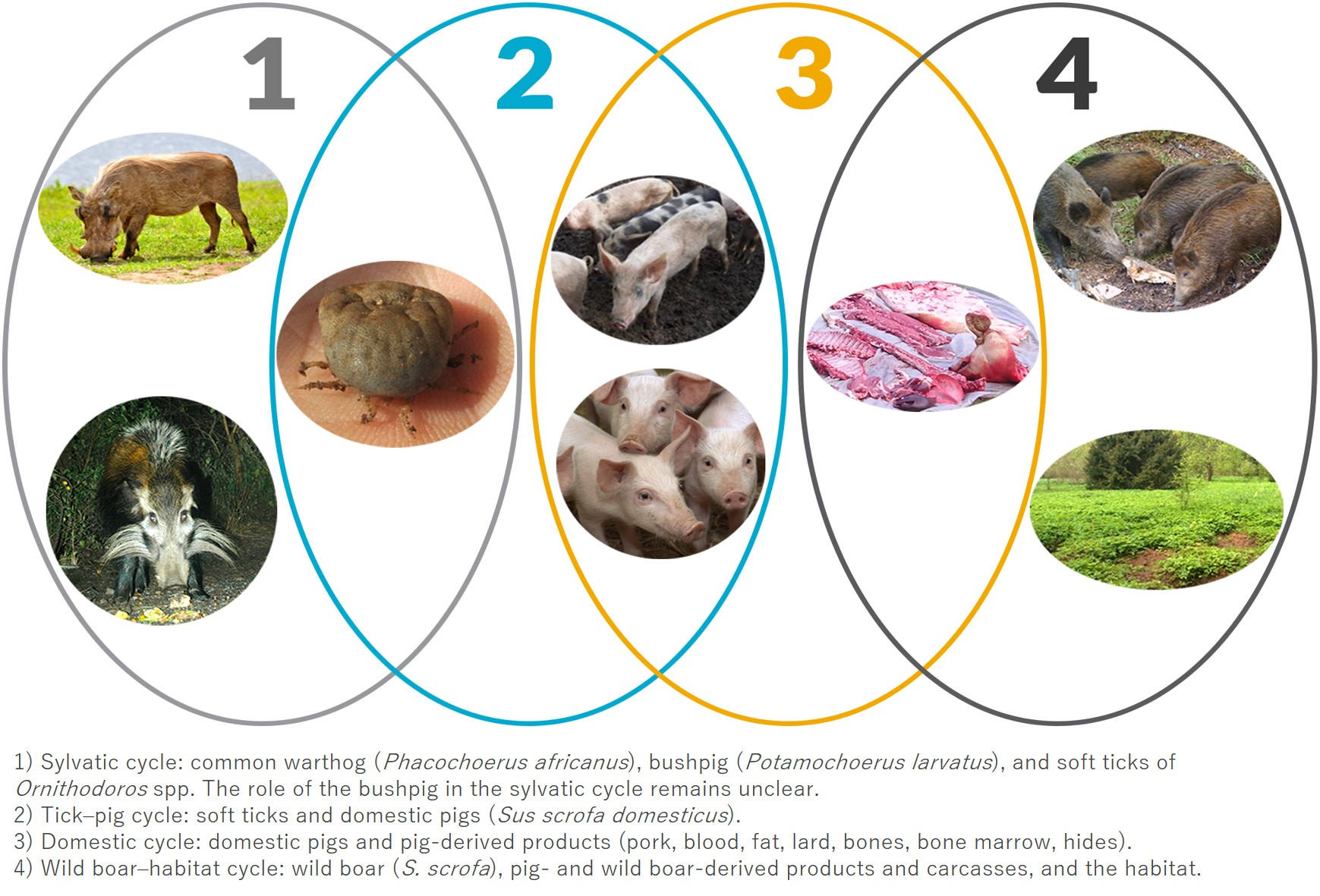
The early detection dilemma
ASF may go unnoticed until mortality becomes significantly raised several weeks after being introduced into domestic pig and wild boar populations, as observed under field conditions [2, 3]. However, improved passive surveillance, including targeted sampling and testing of dead animals, has been shown to promote early detection of ASF [3]. Paradoxically, effective surveillance that enables early detection of ASF before the occurrence of a large number of pig deaths, in combination with the rather low contagiousness, creates a dilemma in justifying the drastic culling of all animals. As a result of this dilemma, and building on an improved understanding of ASF epidemiology and biosecurity, partial stamping out has been discussed, and used under specific circumstances. Fit-for-purpose surveillance and control strategies are therefore essential.
Persistence triangle
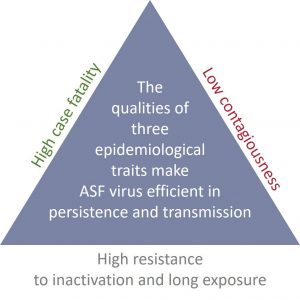
The combination of a high case-fatality rate and resistance to inactivation ensures long-term virus persistence in animal carcasses and the environment; meanwhile, the relatively low contagion rate prevents complete depopulation of the host population (Fig. 2). The interaction of these three parameters maximises both local persistence and constant geographical spread, making the eradication of ASF in natural habitats challenging in the absence of other control tools, e.g. vaccination [1].
http://dx.doi.org/10.20506/bull.2020.1.3121
References
- Chenais E., Depner K., Guberti V., Dietze K., Viltrop A. & Ståhl K. (2019). – Epidemiological considerations on African swine fever in Europe 2014–2018. Porc. Health Manag., 5 (1), 6. https://doi.org/10.1186/s40813-018-0109-2.
- Lamberga K., Seržants M. & Oļševskis E. (2018). – African swine fever outbreak investigations in a large commercial pig farm in Latvia: a case report. Berl. Münch. Tierärztl. Wochenschr. https://doi.org/10.2376/0005-9366-18031.
- Zani L., Dietze K., Dimova Z., Forth J.H., Denev D., Depner K. & Alexandrov T. (2019). – African swine fever in a Bulgarian backyard farm: a case report. Vet. Sci., 2019 (6) 94. https://doi.org/10.3390/vetsci6040094.
- Oļševskis E., Guberti V., Seržants M., Westergaard J., Gallardo C., Rodze I. & Depner K. (2016). – African swine fever virus introduction into the EU in 2014: experience of Latvia. Res. Vet. Sci., 105, 28–30. https://doi.org/10.1016/j.rvsc.2016.01.006.
- Chenais E., Ståhl K., Guberti V. & Depner K. (2018). – Identification of wild boar-habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis., 24 (4), 810–812. https://doi.org/10.3201/eid2404.172127.












