Dossier Posted on 2021-03-08 08:50:12
Rift Valley fever and the challenges of remaining fully prepared for this periodic emergency
Keywords
Authors
Baptiste Dungu (1)* & Assaf Anyamba (2)
(1) Chief Executive Officer, Onderstepoort Biological Products Ltd, Onderstepoort, Pretoria, 0110, South Africa.
(2) Research scientist, Sciences and Exploration Directorate, National Aeronautics and Space Administration (NASA), Greenbelt, MD 2077, United States of America.
* Corresponding author: badungu@gmail.com
The designations and denominations employed and the presentation of the material in this article do not imply the expression of any opinion whatsoever on the part of the OIE concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries.
The views expressed in this article are solely the responsibility of the author(s). The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by the OIE in preference to others of a similar nature that are not mentioned.
The prevention, control and mitigation of RVF in endemic countries and regions require effective surveillance measures, both passive and active. While passive RVF surveillance relies on general animal health surveillance conducted by Veterinary Services, including reports from livestock keepers and other affected sectors, active surveillance includes measures taken by Veterinary Services to specifically monitor the possible circulation of the RVF virus, and also to collect and analyse data on factors that directly influence the occurrence of RVF, such as climate and competent insect-vector pressure. Control measures thus consist of medical and sanitary prophylaxis. Medical prophylaxis consists of vaccination and the implementation of an appropriate vaccination strategy. Sanitary prophylaxis focuses on climate surveillance systems that feed early-warning systems. It uses these early-warning systems to conduct targeted vector surveillance and control and, when at-risk areas have been identified, puts livestock movement controls and zoning in place to mitigate the risk of human and animal outbreaks.
Coordination between the human and animal health sectors is of the utmost importance
At present, RVF tends to be first noticed when human cases appear. This must change. Measures that alert both the veterinary and public health sectors when RVF first appears in livestock have the potential to greatly mitigate the disease’s impacts on both animal and human health.
Climate surveillance and early-warning systems
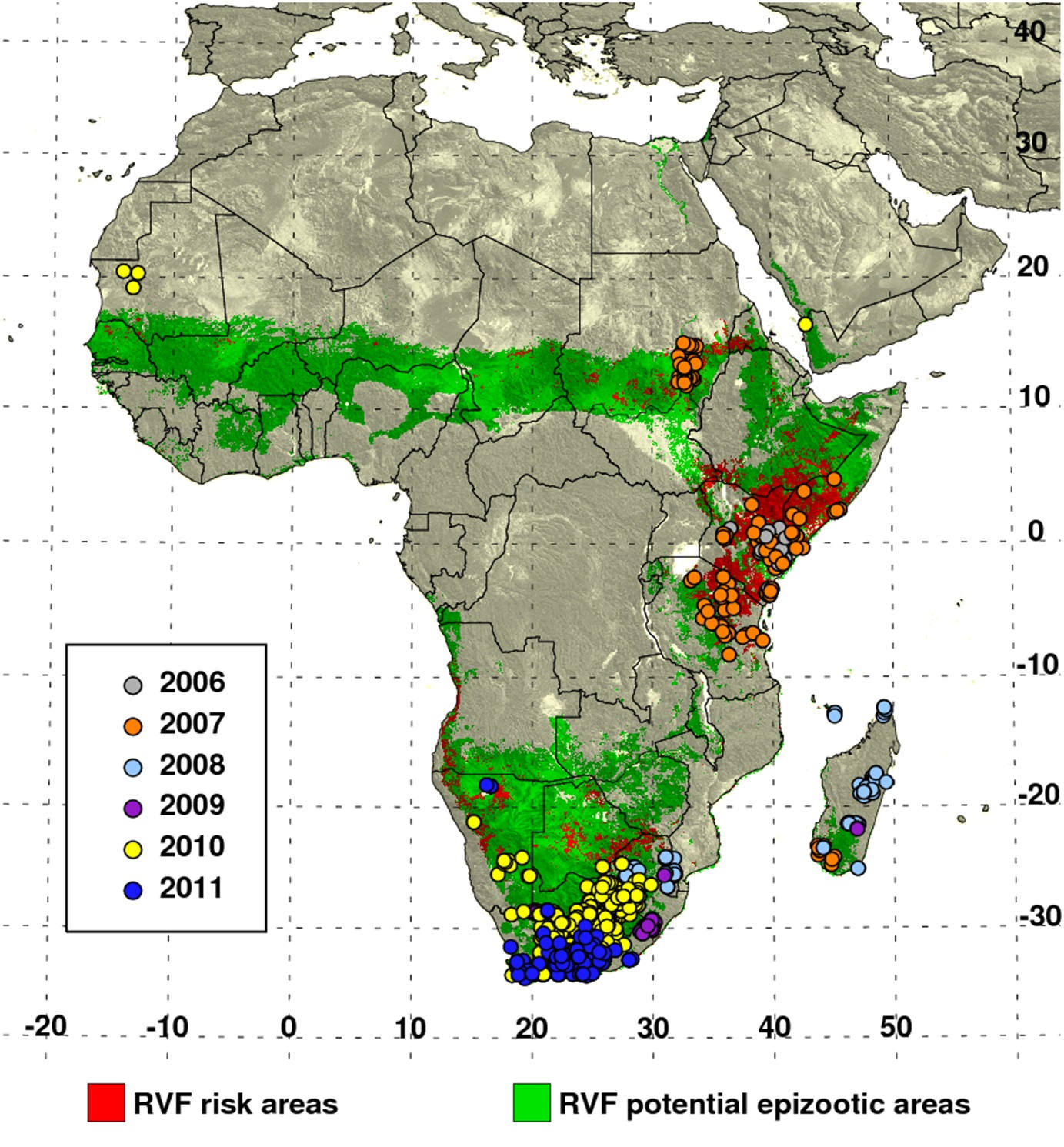
Current early-warning systems gather data on various climate measurements, including sea-surface temperatures as an indicator of the phase and amplitude of El Niño/the Southern Oscillation (ENSO), rainfall, and vegetation conditions. The data are then used to map areas at potential risk of outbreaks [1] (Fig. 1). These early-warning systems can provide three to six months’ lead time before a possible outbreak (Fig. 2). Such early warnings need to be accompanied by on-the-ground vector surveillance and control in areas deemed to be at potential risk, and by vaccination and public awareness campaigns [2].
In addition, emergency preparedness and resilience during inter-epidemic periods is vital. Under changing climate conditions, accompanied by extreme rainfall, the risk of outbreaks is elevated [3]. It is important to employ a risk-based approach to control strategies, including seasonally and geographically targeted vaccination and health promotion campaigns.
Vaccination strategy
The RVF livestock vaccines currently available have clearly demonstrated their effectiveness in controlling RVF in enzootic and epizootic situations. At present, vaccination approaches to RVF [4] are still limited and should be expanded to other endemic and at-risk countries. The costs of not vaccinating, shown by a number of recent outbreaks, should demonstrate the need for national and regional vaccination strategies, which may include the establishment of regional vaccine banks.
Conclusions
Countries need to pay attention to early-warning systems and implement vector control, while establishing effective RVF vaccination strategies. Regional approaches to RVF control, which should also involve public health, are of the utmost importance in high-risk areas if there is to be an efficient response to RVF alerts and outbreaks.
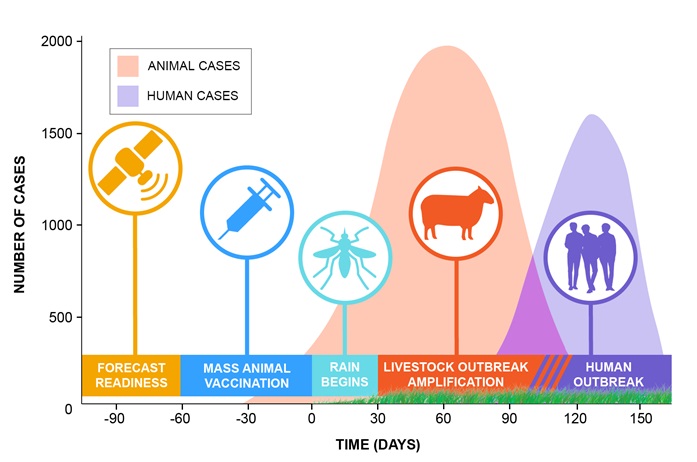
Concept by Assaf Anyamba, design and art by Heidi Tubbs, Universities Space Research Association (USRA) & NASA/Goddard Space Flight Center.
http://dx.doi.org/10.20506/bull.2020.2.3151
References
- Anyamba A., Chretien J.P., Small J., Tucker C.J., Formenty P.B., Richardson J.H., Britch S.C., Schnabel D.C., Erickson R.L. & Linthicum K.J. (2009). – Prediction of a Rift Valley fever outbreak. Proc. Nat. Acad. Sci., 106 (3), 955–959. https://doi.org/10.1073/pnas.0806490106.
- Anyamba A., Linthicum K.J., Small J.S., Britch S.C., Pak E., de La Rocque S., Formenty P., Hightower A.W., Breiman R.F., Chretien J.P., Tucker C.J., Schnabel D., Sang R., Haagsma K., Latham M., Lewandowski H.B., Osman Magdi S., Mohamed A., Nguku P.M., Reynes J.M. & Swanepoel R. (2010). – Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006–2008 and possible vector control strategies. Am. J. Trop. Med. Hyg., 83 (2 Suppl), S43–S51. https://doi.org/10.4269/ajtmh.2010.09-0289.
- Martin V., Chevalier V., Ceccato P., Anyamba A., De Simone L., Lubroth J., de La Rocque S. & Domenech J. (2008).– The impact of climate change on the epidemiology and control of Rift Valley fever. In S. de La Rocque, G. Hendrickx & S. Morand (eds). Climate change: impact on the epidemiology and control of animal diseasesRev. Sci. Tech. Off. Int. Epiz, 27 (2), 413–426. https://doi.org/10.20506/rst.27.2.1802.
- Dungu B., Lubisi B.A. & Ikegami T. (2018). – Rift Valley fever vaccines: current and future needs. Curr. Opin. Virol., 29, 8–15. https://doi.org/10.1016/j.coviro.2018.02.001.